We read the marking key
You get the marks
You get the marks
Stop Guessing. Start Dominating.
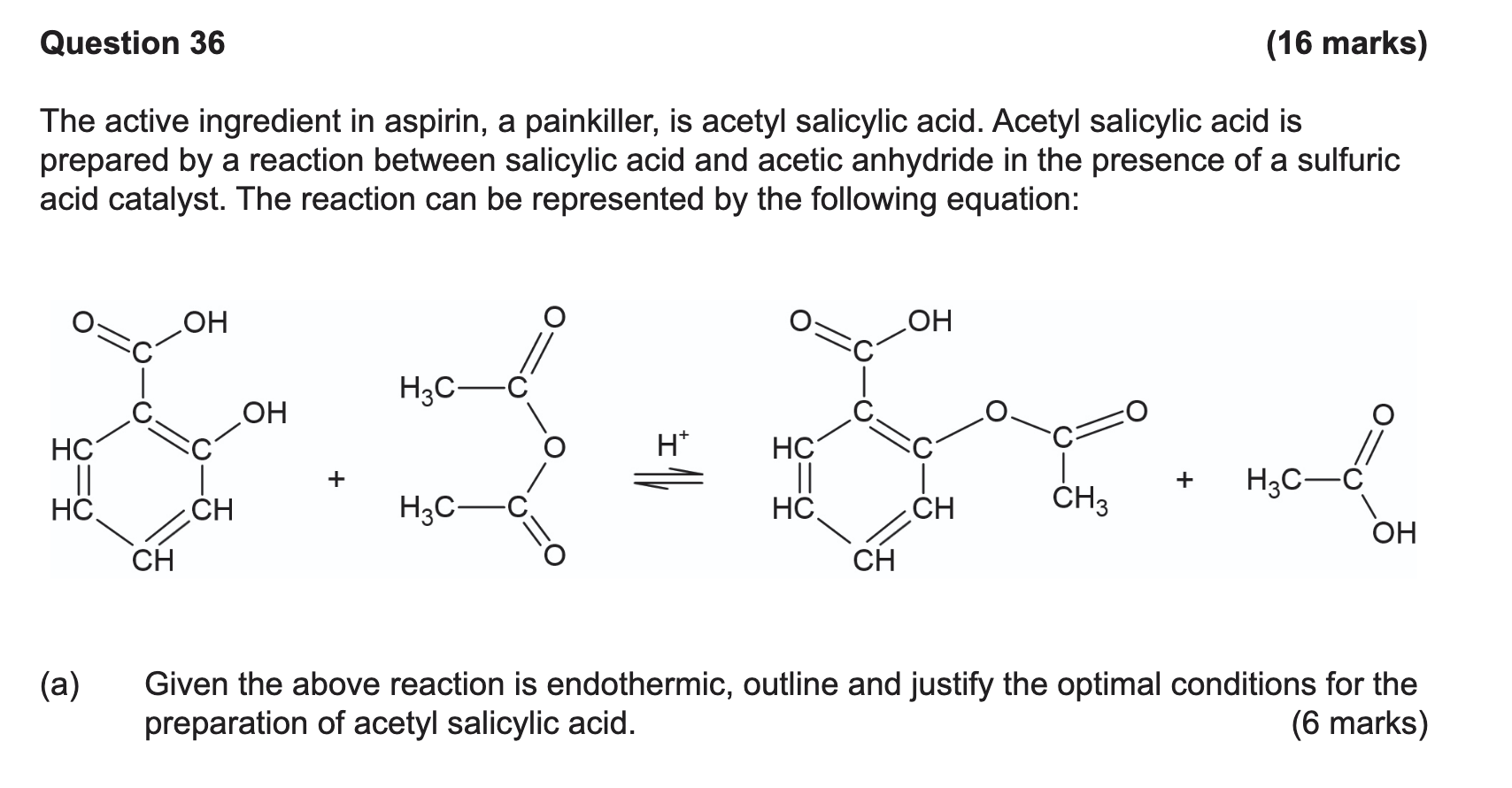
- For a reaction to take place particles must collide with
sufficent force and favourable orientation
- As temperature increases so does the mean translational kinetic energy
- They will collide with a greater frequency and energy
- Increased frequency of successful collisions and increased reaction rate
- As temperature increases so does the mean translational kinetic energy
- They will collide with a greater frequency and energy
- Increased frequency of successful collisions and increased reaction rate